SOIL
TRIANGLE
Soil
Textural Triangle
Use
a textural triangle to determine textural classes of soil if given
the percent of two of the soil separates
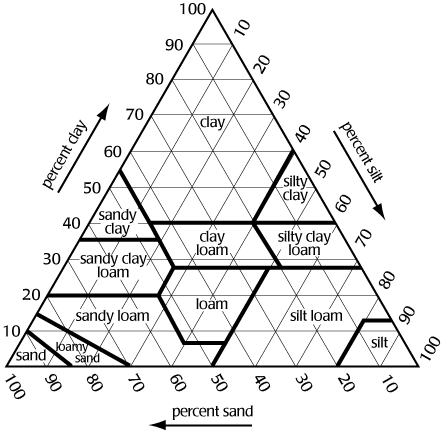
Figure
1 shows a textural triangle. The bottom of the triangle is labeled
sand, the left side silt, and the right side clay. Each side is divided
100 segments. If we know the percentage of sand, silt, and clay in
the soil, we can determine its texture. For example, if a soil is
40% sand, 30% silt, and 30% clay, the texture is clay loam.
Note:
To determine the texture, lines from the sides must be extended in
the correct direction. The triangle is equilateral i.e., all angles
are 60 degrees. Proceed as follows:
clay
extend line horizontal from the % clay
i.e., parallel with side labeled sand
silt
extend line downward from % silt at 60 degrees
i.e., parallel with side labeled clay
sand
extend line upward from % sand at 120 degrees
i.e., parallel with side labeled silt
Back
to Top of Page
Mineral
versus Organic Soil Material
Mineral
soil material is defined by exclusion from either of these two requirements
that define organic soils:
1.
Organic soil has more than 20 percent organic carbon (about 35 percent
humus) if it is never saturated with water more than a few days.
2.
Organic soil has water saturation periods (unless drained by people)
and has:
a.
At least 12 percent organic carbon (about 21 percent humus) if the
soil has no clay, or
b. has at least 18 percent organic carbon (about 31 percent humus)
if the soil has 60 percent or more clay, or
c. has an intermediate, proportional amount of organic carbon for
intermediate amounts of clay.
Soils
with less organic carbon than these amounts are called mineral soils.
Back
to Top of Page
Chelates
Chelates
complex organic molecules used to maintain the plant availability of
micronutrients.
Chelates
prevent the micronutrient cation from interacting with the soil and
forming insoluble compounds with soil constituents.
Effectiveness
of the chelate is related to the stability of the chelate at different
pH levels. Chelates should be chosen dependent on the soil pH. For example:
EDTA
chelates Fe at a pH < 6.3
DTPA
chelates Fe at a pH < 7.5
EDDHA
chelates Fe over a pH range of 4 9
If
pH = 8.0 then only EDDHA will be effective. If pH = 6.2 then all will
be effective.
For
soil applications much lower rates of chelated micronutrients are needed
to supply the plant than inorganic sources. This is because the availability
is higher. In some cases the application of the non chelated micronutrient
is so rapid that it is ineffective as a nutrient source.
Chelates
are generally more expensive than inorganic sources. Applications should
be as near the point of crop demand as possible.
Inorganic
sources and chelates are equally effective for foliar applications.
Back
to Top of Page
Ph
The pH Defined
Soil
pH is the negative logarithm of the active hydrogen ion (H+)
concentration in solution. When water (HOH) ionizes to H+
and OH- (a neutral solution), both H+ and OH-
are in concentrations of 10-7 mole* per liter. The [ ] =
concentration.
HOH
= H+ + OH-
[H+][OH-]
---------- = 1 X 10-14 , [H+] = [OH-]
= 1 x 10-7
[HOH]
Thus, the negative logarithm of [H+] is 7, or PH 7. When
the H+ concentration is greater (more acidic), such as 10-4
mole per liter, the pH is lower (e.g., pH 4). In basic solution the
OH concentration exceeds the H+ concentration. The product
of the H+ and OH- concentrations equals 10-14
mole per liter. When H+ is 10-5, OH-
is 10-9, for example. (The weight and volume units in chemistry
are always expressed in the centimeter- gram-second system, and conversions
to the U.S. units are not applicable here.)
"A
mole is one molecular weight of ion or of the molecule.
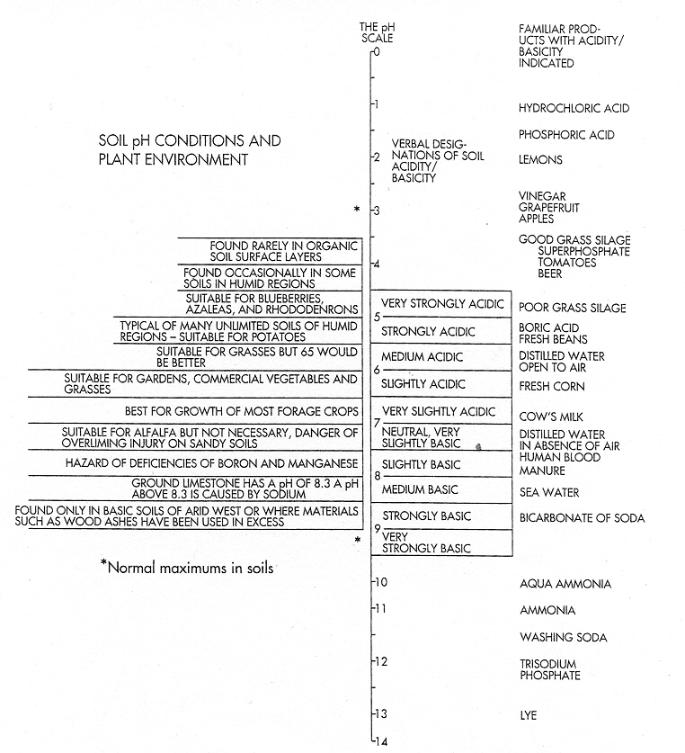
FIGURE
5-8 The entire pH scale ranges from 0 to 14, but soils under field conditions
vary between pH 3.5 and 10.0. Few soils have pHs outside this range.
in general, most plants are best suited to a pH of 5.5 on organic soils
and a pH of 6.5 on mineral soils, (Source: Adapted from Winston A. Way,
"The Whys and Hows of Liming," University of Vermont Brieflet
997, 1968.)
Back
to Top of Page


