Soil
colloids, clay minerals and soil organic matter account for cation
exchange properties of soils. See Chapter 5 of text for discussion
of soil colloids, structural features, and other characteristics of
soil colloids. Soil organic matter means the organic fraction of the
soil but does not include undecayed plant and animal residues. Estimates
of the average age of the carbon in soil organic matter, based on
radiocarbon dating, varies from a few hundred years to more than 25,000
years. (See page 217 in text.) Soil organic matter does not consist
primarily of recent crop residues. It is a recalcitrant mixture of
organic "residuals" that resist decomposition. Generally,
it takes many years to change the organic matter content of a soil
from its current "equilibrium" value. Tillage is the major
agronomic practice that affects soil organic matter content. Reducing
tillage is the most effective way to maintain or attempt to build
back organic matter content that has been severely depressed due to
intensive or non conserving agricultural practices. However, climate
exerts a natural control or limit as to the amount of soil organic
matter that can be achieved and sustained.
Cation
exchange capacity (CEC)
The CEC
of a soil depends upon the amount and type of soil colloids present.
The clay content, the type of clay minerals present, and the organic
matter content determine a soil's CEC.
|
Colloid
|
CEC,
cmol(+) /kg*
|
|
kaolinite
|
3
- 15
|
|
illite
|
20 - 40
|
|
montmorillonite
|
60
- 100
|
|
soil
organic matter, humus, etc.
|
100
- 300
|
*Unit
is centimole of charge per kilogram of colloid; another
common unit for expressing CEC is me/100g (milliequivalents/100
grams). Note that 1me/100g = 1cmolc /kg.
Kaolinite is the dominant clay mineral in soils of this region,
but some soils contain significant amounts of montmorillonite, a clay
mineral of the smectite group.
CEC for
various soils: a typical range of CEC for soils in the state and the
region is 3-5 me/100 g. See page 147 of text for a range of CEC for
a variety of soils from various regions of the U. S. You may encounter
a CEC expressed as "sum of the bases" (Ca+Mg+K+Na) or a
CEC based on "sum of the bases and exchangeable acidity"
(H+Al). For soils such as those in the Piedmont there is usually a
large difference. Generally CEC is not used directly to manage soils
and their fertility. The effect of CEC and its significance in managing
soils is incorporated into management of pH and lime requirement.
The larger the CEC the more buffering capacity a soil will have and
the more lime that will be required to raise the soil pH by a specific
amount, for example, from 5.5 to 6.5. By the same token soils with
a large CEC will have more K supplying power for a given degree of
K saturation. Following proven soil test methods and fertilizer recommendations
is the best way to manage plant nutrient supplies.
Conversion
of me/100 grams to pounds/acre and other facts relating to acreage:
|
Element
|
Factor
|
Example
|
|
Ca
|
400
|
1
me Ca/100 grams = 400 lbs /acre
|
|
K
|
780
|
1
me K/100 grams = 780 lbs/acre
|
|
Mg
|
240
|
1
me Mg/100 grams = 240 lbs/acre
|
|
Na
|
460
|
1 me Na/100 grams= 460 lbs/acre
|
These
conversions are based on an estimate that the surface 6 inch layer
of soil over the area of an acre weighs 2,000,000 pounds. The actual
weight depends upon the soil bulk density which commonly varies from
about 1.3 to 1.7 g/cc. For example, a cubic foot of water weighs 62.4
lbs. If the bulk density of a soil were 1.3 g/cc (1.3 times heavier
than water) then the soil would weight 62.4 x 1.3 or 81.1 lbs/cu.
ft. on a dry weight basis. Soil properties such as clay content and
gravimetric water content are always expressed on a soil dry weight
basis.
Other
useful numbers to remember and examples of how to use them:
1 acre
= 43,560 sq ft
the weight of an acre foot of soil with a bulk density of 1.47
= 43,560 sq ft x 1 ft x 62.4 lbs/cu ft x 1.47 = 4,000,000 lbs
1 part per million (ppm) nitrate N in the top 12 inches of this
soil = 4 lbs/acre
If you had a row spacing of 36 inches,
one row 14,520 feet long would be an acre (43,560 ft2/3 ft);
if
the row spacing were 40 inches,
the row would be 13,068 feet long (43,560/(40/36) or 43,560/3.333).
- 454 grams = 1 pound; 1 ounce = 28.4 grams
- 1 acre inch of water = 3,630 cu ft = 27,154 gallons = 226,512
pounds
- 1 cu ft weighs 62.4 pounds
Base saturation
Back
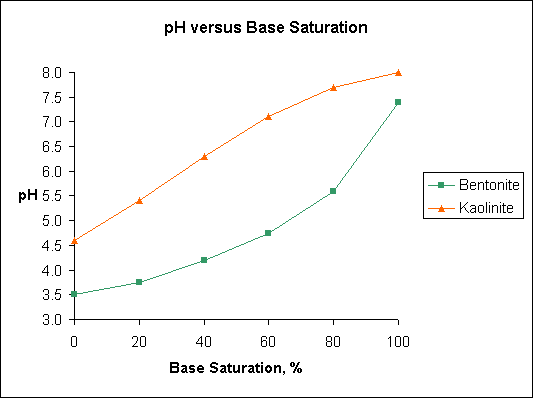
Note
differences in base saturation of kaolinite and bentonite over the
pH range 5.5 to 6.5. Humic acid and illite have pH-base saturation
ralationships similar to bentonite.
The figure "Base Saturation" is based on data published
in Soil Testing and Plant Analysis, Edited by Leo M. Walsh and James
D. Beaton and published by the Soil Science Society of America, Madison,
Wisconsin in 1973. The original data was developed by A. Mehlich and
published in 1943.
Base
saturation is the amount of the CEC that is occupied by the basic
cations such as Ca, K, Mg, and Na. The portion of the CEC that is
occupied by acidic cations, primarily H, Al, and Fe, is called the
"exchangeable acidity". Note differences in base saturation
of kaolinite and bentonite over the pH range 5.5 to 6.5. Humic acid
and illite have pH base saturation relationships similar to bentonite.
Unless
one knows some specific characteristics about a soil such as the dominant
clay minerals, the amount of clay in the soil, and the amount of organic
matter, soil pH will not tell how much is the lime requirement. The
pH is a measure of the amount of hydrogen ion in solution. As is true
of all cationic species, acidic as well as bases, only a small amount
of the total exchangeable ions are present in the soil solution at
one time.
Soil
test methods that have been developed to quickly measure exchangeable
acidity must be relied upon to estimate the amount of lime required
to raise the soil pH to a desired range.